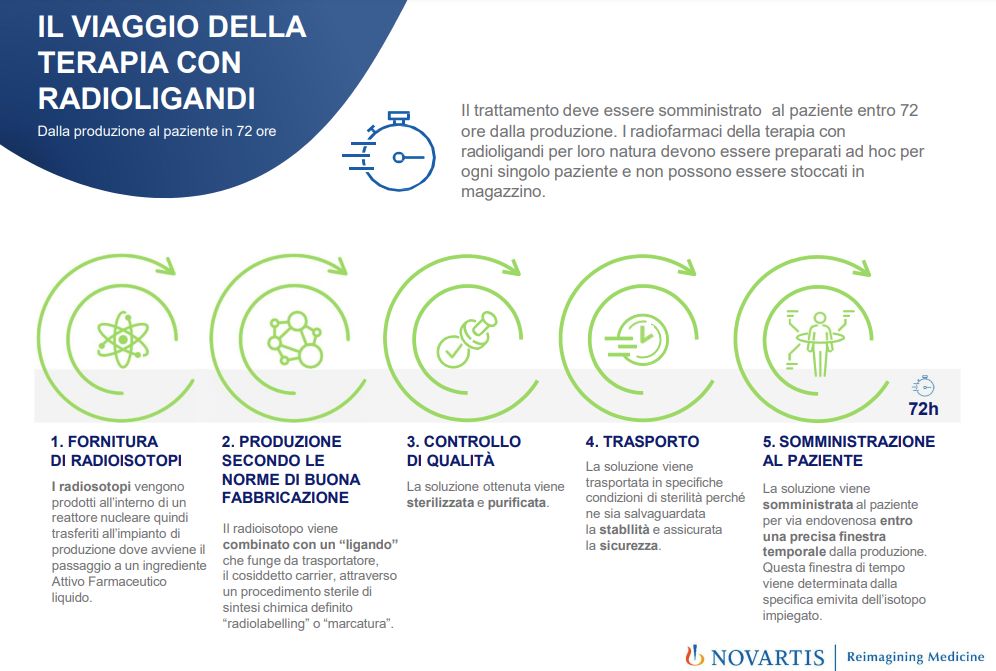
MILAN (ITALPRESS) – With the approval of Lutetium (177Lu) vipivotide tetraxetan for reimbursability by the CDA of the Agenzia Italiana del Farmaco, radioligand-based precision therapy will soon become accessible for Italian patients with progressive metastatic castration-resistant prostate cancer (mCRPC), prostate-specific membrane antigen (PSMA)-positive, who have been treated with androgen receptor (AR) pathway inhibitor and docetaxel- and cabazitaxel-based chemotherapy or who are not candidates for cabazitaxel. An achievement that represents a significant evolution in the treatment of a complex oncologic disease, such as prostate cancer, expanding available therapeutic options and improving survival.Radioligand therapy represents one of the most important technological advances enabling precision and personalized nuclear medicine based on teragnostics. An innovative approach that integrates the diagnostic moment with the therapeutic moment in a single pathway, allowing at the same time to recognize markers of cancer cells that can then become therapeutic targets. “With the introduction of radioligands into clinical practice, a new piece in the most modern precision medicine is introduced. Indeed, radioligand therapies represent a significant innovation in the fight against cancer, as they offer a targeted approach that combines nuclear medicine with the selectivity of personalized therapy. A new and effective therapeutic approach, which also positively impacts patients’ quality of life,” comments Carmine Pinto, Director of Medical Oncology at the Comprehensive Cancer Centre, of the AUSLIRCCS in Reggio Emilia.An innovation that has its roots in Made in Italy research, carried out precisely in Ivrea, a cutting-edge reality of the Novartis Group that developed the first radiopharmaceutical therapies. At the moment, the Ivrea site is one of 4 plants in the world, as well as the only one in Italy, capable of producing and distributing this type of radiopharmaceutical internationally. “The target therapy with radioligands,” says Giuseppe Procopio, Head of Genitourinary Medical Oncology at Fondazione IRCCS Istituto Nazionale dei Tumori in Milan, Italy, “represents a breakthrough for patients with advanced prostate cancer, offering new hope to those who have already received previous treatments. This therapy selectively targets cancer cells that express the PSMA receptor on the surface, which is present in more than 80 percent of patients with metastatic disease. The Phase III Vision clinical trial demonstrated a 38 percent reduction in the risk of death compared with the best standard of care. Lutetium (177Lu) represents an important therapeutic solution that can significantly improve both the quality and life expectancy of patients, opening up new therapeutic opportunities. “Prostate cancer is the most frequent cancer among men in Western countries and in Italy represents 29.9 percent of all male cancers, with 485,000 men living with this cancer diagnosis and 40,192 new cases in 2024. The metastatic castration-resistant form is the most advanced stage of the disease, with a median 5-year survival of no more than 30 percent.Novartis expresses satisfaction with the agreement signed with AIFA at the conclusion of a responsible collaboration pathway, which has placed an appropriate value on this therapy and will allow patients and the NHS to have access to this important therapeutic innovation in a sustainable manner.With this approval, Novartis confirms its commitment to the development of innovative cancer therapies. “Our mission is to transform science into affordable therapeutic solutions, improving patients’ life prospects,” comments Paola Coco, Medical Affairs Head Novartis.As pioneers in the development of radioligand therapies, we have transformed years of research and investment into treatments that offer real hope for patients. Access to this treatment has been made possible through close collaboration with regulatory authorities. “Given the administrative time required to publish the agreement in the Official Gazette, Lutetium (177Lu) vipivotide tetraxetan is expected to be available on a reimbursable basis from March.-photo press office Novartis – (ITALPRESS).